-
PepGen Announces IND-Enabling Preclinical Data Supporting Progression of PGN-EDODM1 into Clinical Studies
Source: Nasdaq GlobeNewswire / 07 Dec 2022 08:00:01 America/New_York
- PGN-EDODM1 was well-tolerated in acute GLP studies in rodents and non-human primates (NHPs) at clinically relevant doses-
- Studies conducted in patient cells indicate that PGN-EDODM1, a CUG-targeting peptide-phosphorodiamidate morpholino oligonucleotide (PPMO) conjugate, did not degrade DMPK transcripts, a potentially important safety benefit, while reducing toxic nuclear foci and correcting mis-splicing -
- Off-target effects were not observed in other transcripts containing more than 10 CUG repeats in the HSALR mouse model of disease -
- PepGen anticipates initiating a single ascending dose clinical trial in DM1 patients in the first half of 2023 -
BOSTON, Dec. 07, 2022 (GLOBE NEWSWIRE) -- PepGen Inc. (Nasdaq: PEPG), a clinical-stage biotechnology company advancing the next generation of oligonucleotide therapies with the goal of transforming the treatment of severe neuromuscular and neurologic diseases, today announced new preclinical data supporting the progression of PGN-EDODM1, PepGen’s product candidate in development for the treatment of myotonic dystrophy type 1 (DM1), into clinical trials. PGN-EDODM1 leverages PepGen’s Enhanced Delivery Oligonucleotide (EDO) technology and consists of the Company’s proprietary cell-penetrating peptide conjugated to a steric blocking oligonucleotide cargo. This well-characterized therapeutic oligonucleotide is designed to bind to the CUG repeat expansion hairpin loops in the DMPK transcripts of people with DM1, and thus subsequently correct the downstream transcript mis-splicing events that lead to myotonia and cardiac dysfunction.
“We have previously shown that PGN-EDODM1 can achieve greater than 60% correction of the mis-splicing events in a murine model of DM1, resulting in a complete reversal of myotonia. This correction in mis-splicing was observed to be highly durable, with activity sustained through 24 weeks following a single dose. Furthermore, results from our recent Phase 1 clinical trial of PGN-EDO51 in healthy volunteers suggest that our EDO technology has the potential to deliver therapeutic levels of the PGN-EDODM1 oligonucleotide to human muscle,” stated James McArthur, Ph.D., President and CEO of PepGen. “We are now pleased to provide an update on the safety profile of PGN-EDODM1, having completed IND-enabling preclinical studies in multiple models. Notably, we observed that this product candidate was well-tolerated in acute GLP studies conducted in rodents and NHPs at what we believe are clinically relevant doses. In addition, while we have observed that PGN-EDODM1 corrects mis-splicing in cells with both long and short CTG repeats, our studies indicate that this product candidate does not mediate the degradation of DMPK transcripts. Finally, no off-target effects were observed in other transcripts containing more than 10 CUG repeats in a DM1 mouse model of disease.”
Dr. McArthur continued, “We are highly encouraged by the results seen in these IND-enabling studies, and are excited by the potential of PGN-EDODM1 to address the root cause of this debilitating disease without degrading DMPK. We believe that PGN-EDODM1 offers a targeted approach for the treatment of DM1, without the risk of potential confounding effects due to the knockdown of DMPK. There are no approved, disease-modifying therapeutics currently available to those living with DM1, and we look forward to advancing PGN-EDODM1 for this patient population. We anticipate initiating a single ascending clinical trial in DM1 patients in the first half of 2023.”
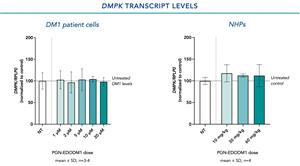
DMPK Transcript Levels Following Treatment with PGN-EDODM1
Building on previous work, where PGN-EDODM1 was observed to exhibit high levels of activity in preclinical models with both long and short CTG repeats, PepGen has completed a number of rigorous studies that support a well-tolerated safety profile for this product candidate. In an in vitro study, immortalized myoblasts from a DM1 patient with 2,600 CTG repeats were differentiated for 4 days to myotubes and treated for 24 hours with PGN-EDODM1 at a range of concentrations between 1 and 20 µM. DMPK transcript levels were evaluated by qPCR and normalized to RPLP0. In NHPs, three doses of 10, 30 or 60 mg/kg of PGN-EDODM1 were administered every two weeks. One week following the final dose, DMPK transcript levels were evaluated by qPCR and normalized to RPLP0.
- In both DM1 patient cells and in NHPs, mean DMPK transcript levels remained unchanged relative to an untreated control.
- We believe these results indicate that PGN-EDODM1, as designed, does not target DMPK transcripts for degradation – a potentially important safety benefit that would not carry the risk of possible confounding effects due to the knockdown of DMPK.
Figure 1. No significant changes in mean DMPK transcript levels were observed in DM1 patient cells or in NHPs.
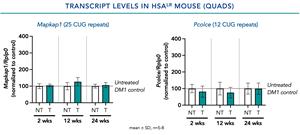
PGN-EDODM1 Safety and Tolerability Profile
- There was no evidence of off-target effects in any of the preclinical studies conducted.
- In a single-dose study of PGN-EDODM1 conducted in a mouse model of DM1, no short- or long-term impacts through 24 weeks were observed on the levels of other transcripts containing more than 10 CUG repeats.
- PGN-EDODM1 was observed to be well-tolerated in NHPs through 90 mg/kg in acute GLP toxicology studies.
- In addition, there were no adverse effects on kidney, liver or cardiovascular function.
- We believe that the totality of the data obtained from these preclinical and IND-enabling studies highlights a potentially favorable safety profile for PGN-EDODM1.
Figure 2. No significant impact was observed on the mean levels of other transcripts containing more than 10 CUG repeats.
PGN-EDODM1 Next Steps
PepGen anticipates initiating a single ascending dose clinical trial of PGN-EDODM1 in DM1 patients in the first half of 2023, with the goal of delivering safety, tolerability and activity readouts in 2024.
About Myotonic Dystrophy Type 1 (DM1)
DM1 is a monogenic, autosomal dominant, progressive multisystem disorder primarily affecting skeletal, cardiac and smooth muscle as well as the CNS, resulting in significant physical, cognitive and behavioral impairments and disability. The burden of disease is significant, and many people living with DM1 have a shortened lifespan. DM1 is caused by an abnormal trinucleotide repeat expansion in a region of the DMPK gene. DM1 is estimated to affect approximately 40,000 patients in the United States, 75,000 patients in Europe and 15,000 patients in Japan. There are currently no approved therapies for the treatment of DM1.
About PepGen
PepGen Inc. is a clinical-stage biotechnology company advancing the next-generation of oligonucleotide therapies with the goal of transforming the treatment of severe neuromuscular and neurological diseases. PepGen’s Enhanced Delivery Oligonucleotide, or EDO, platform is founded on over a decade of research and development and leverages cell-penetrating peptides to improve the uptake and activity of conjugated oligonucleotide therapeutics. Using these EDO peptides, we are generating a pipeline of oligonucleotide therapeutic candidates that target the root cause of serious diseases.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts,” “goal,” “intends,” “may,” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. Any such statements in this press release that are not statements of historical fact may be deemed to be forward-looking statements. These forward-looking statements include, without limitation, statements about our clinical and preclinical programs, product candidates, including their planned development and therapeutic potential, plans for future development and clinical trials in our programs, achievement of milestones, and corporate and clinical/preclinical strategies.
Any forward-looking statements in this press release are based on current expectations, estimates and projections only as of the date of this release and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to that we may fail to successfully complete preclinical studies and clinical trials of our product candidates or to obtain regulatory approval for marketing of such products, including PGN-EDODM1; initial preclinical study or clinical trial results for one or more of our product candidates, including PGN-EDODM1 may not be predictive of future trial results for such candidates; our product candidates, including PGN-EDODM1 may not be safe and effective; there may be delays in regulatory clearance or changes in regulatory framework that are out of our control; our estimation of addressable markets of our product candidates may be inaccurate; more efficient competitors or more effective competing treatments may emerge; we may be involved in disputes surrounding the use of our intellectual property crucial to our success; earlier-stage trial results may not be predictive of later stage trial outcomes; and we are dependent on third parties for some or all aspects of our product manufacturing, research and preclinical and clinical testing. Additional risks concerning PepGen’s programs and operations are described in its most recent quarterly report on Form 10-Q on file with the SEC. PepGen explicitly disclaims any obligation to update any forward-looking statements except to the extent required by law.
Investor Contact
Laurence Watts
Gilmartin Group
Laurence@gilmartinir.comMedia Contact
Gwendolyn Schanker
LifeSci Communications
gschanker@lifescicomms.comGraphics accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/3d911ae5-64a0-4e7a-bc73-218279f297f1https://www.globenewswire.com/NewsRoom/AttachmentNg/16f0d42a-252a-4f58-bcd0-d0c20dd5b2ba